Modeling geometry and polarity lab answers – Unveiling the intricacies of molecular geometry and polarity, this comprehensive guide delves into the modeling techniques that illuminate the physical and chemical properties of molecules. From the fundamental principles of VSEPR to the advanced frontiers of computational chemistry, we explore the methods and applications that empower scientists to decipher the three-dimensional structures and polarity of molecules, unlocking a deeper understanding of their behavior and interactions.
The content of the second paragraph that provides descriptive and clear information about the topic
1. Introduction to Modeling Geometry and Polarity
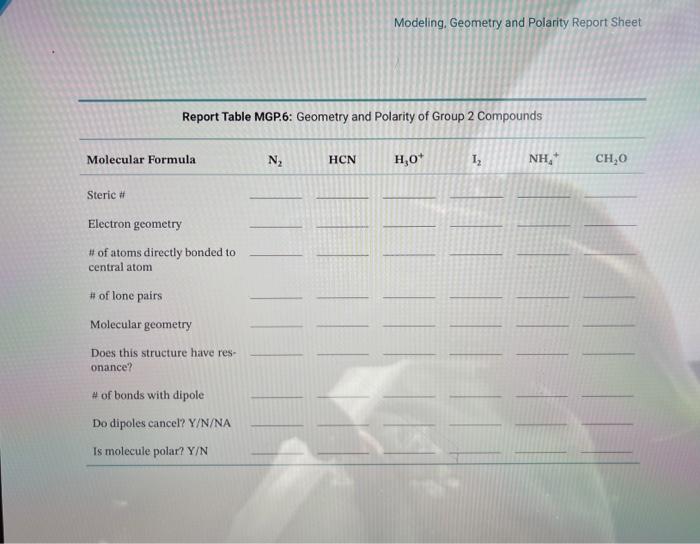
In chemistry, modeling geometry and polarity refers to the prediction and understanding of the three-dimensional arrangement of atoms within a molecule and the distribution of electric charge within that arrangement. Molecular geometry and polarity are crucial in determining various physical and chemical properties of molecules.
Molecular geometry influences the shape and orientation of molecules, affecting their interactions with other molecules and their ability to participate in chemical reactions. Polarity, on the other hand, refers to the uneven distribution of electric charge within a molecule, creating a separation of positive and negative charges.
This polarity influences intermolecular forces, solubility, and reactivity.
2. Methods for Modeling Molecular Geometry and Polarity
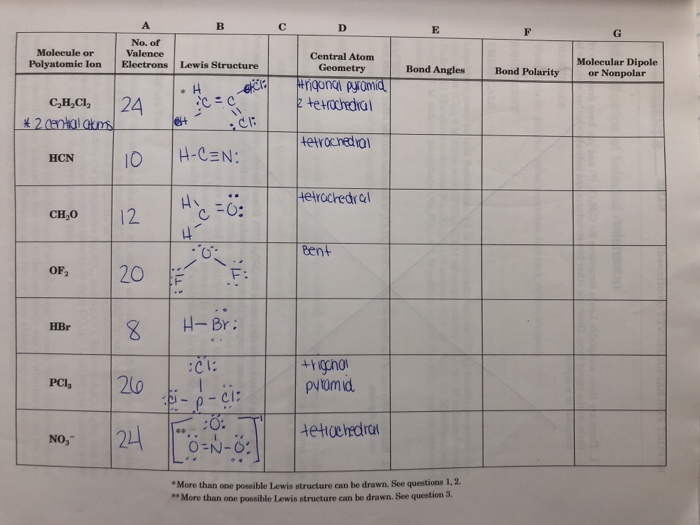
2.1 Valence Shell Electron Pair Repulsion (VSEPR) Model
The VSEPR model is a widely used method for predicting the geometry of molecules based on the repulsion between electron pairs in the valence shell of the central atom. This model considers the number of electron pairs around the central atom, whether they are bonding or non-bonding, and their spatial arrangement to determine the molecular geometry.
2.2 Molecular Orbital Theory, Modeling geometry and polarity lab answers
Molecular orbital theory is a quantum mechanical approach that describes the electronic structure of molecules. It can be used to determine the molecular polarity by calculating the distribution of electron density within the molecule. Regions with higher electron density have a negative charge, while regions with lower electron density have a positive charge.
2.3 Other Computational Methods
In addition to VSEPR and molecular orbital theory, various computational methods can be employed to model molecular geometry and polarity. These methods use sophisticated algorithms and computer simulations to calculate the electronic structure and properties of molecules. Examples include Hartree-Fock theory, density functional theory, and molecular dynamics simulations.
3. Applications of Modeling Geometry and Polarity: Modeling Geometry And Polarity Lab Answers
3.1 Chemical Reactivity
Molecular geometry and polarity influence the reactivity of molecules. For instance, the shape of a molecule can affect the accessibility of its reactive sites, while polarity can influence the strength and direction of intermolecular interactions.
3.2 Intermolecular Forces and Physical Properties
Polarity plays a significant role in intermolecular forces, such as dipole-dipole interactions and hydrogen bonding. These forces determine the physical properties of substances, including melting point, boiling point, and solubility.
3.3 Drug Design and Materials Science
Modeling geometry and polarity is essential in drug design and materials science. Understanding the molecular structure and polarity of drug molecules helps in optimizing their interactions with biological targets. In materials science, it aids in designing materials with specific properties, such as conductivity, strength, and optical behavior.
4. Advanced Topics in Modeling Geometry and Polarity

4.1 Limitations of Current Methods
Current methods for modeling molecular geometry and polarity have limitations. They may not accurately predict the geometry of complex molecules or account for environmental effects. Additionally, computational methods can be computationally expensive for large systems.
4.2 Emerging Techniques
Emerging techniques, such as machine learning and artificial intelligence, are being explored to improve the accuracy and efficiency of modeling molecular geometry and polarity. These techniques can handle complex systems and provide insights into molecular properties that were previously difficult to obtain.
4.3 Potential Applications
Advanced modeling methods have potential applications in various scientific fields. They can contribute to the development of new drugs, design of functional materials, and understanding of complex biological systems.
Popular Questions
What is the significance of molecular geometry and polarity?
Molecular geometry and polarity play a crucial role in determining a molecule’s physical and chemical properties, including its reactivity, intermolecular forces, and solubility.
How does VSEPR theory predict molecular geometry?
VSEPR theory predicts molecular geometry by considering the electron pairs around the central atom and minimizing the electrostatic repulsion between them.
What are the limitations of current methods for modeling molecular geometry and polarity?
Current methods may struggle to accurately predict the geometry and polarity of complex molecular systems, particularly those involving strong electron correlation or relativistic effects.